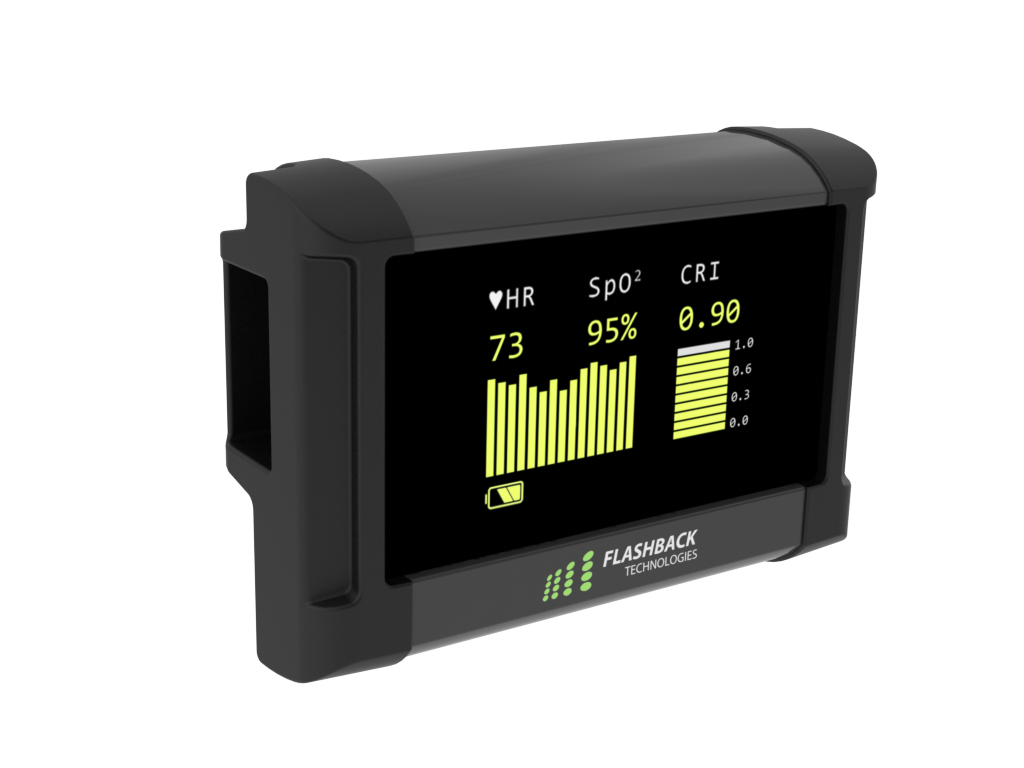
Our team was commissioned to give physicality to an innovative, blood monitoring algorithm that existed on a small set of printed circuit boards. The device would calculate and display heart rate, blood-oxygen (SpO2) and the Compensatory Reserve Index (CRI) using one, simple finger-clip sensor input. CRI is a breakthrough hemodynamic parameter that provides real-time, noninvasive indication of changes in intravascular volume relative to the individual patient’s response to blood loss. Put simply, how much blood is in the patient as a percentage of maximum.
DESIGN CONSIDERATIONS:
Design Enclosure
2.4” Display
High Impact Body
Sterilizable Plastic Materials
IP33-rated
MIL-spec
2-shot Injection Mold
Two-button Operation
One replaceable AA Battery
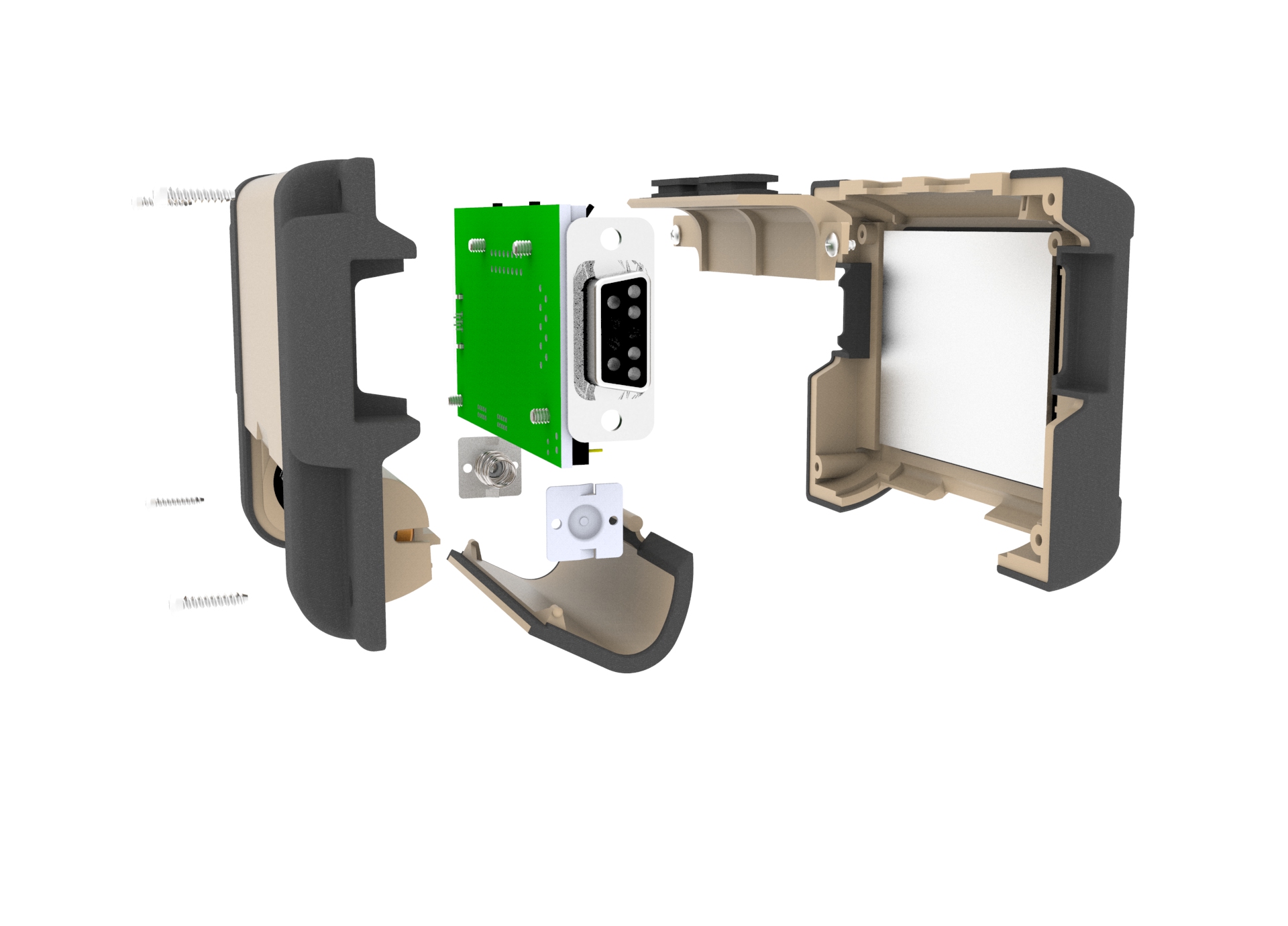
Hinged battery door
Sealed Battery Compartment
Weatherproof Connectors:DB9 and Micro USB
Insert-molded Lanyard Clip
Possible Secondary Belt Clip
2 Color Schemes
Context
This device was first targeted for use in a military medevac environment. Several stringent requirements had to be met to endure the testing and approval process.
Early Concepts
CONCEPT 1
CONCEPT 2
CONCEPT 3
Concept Refinement Phase - cONCEPT a
Concept Refinement Phase - cONCEPT b
Initial Prototype - rEVISION a
20 prototypes of Revision A were built for field testing and manufacturing viability.
Final Prototype - rEVISION b for Military Testing
Feedback from Revision A resulted in several changes: battery door sealing strategy, button size, and the addition of a insert-molded lanyard clip.
Results:
On July 24, 2018, Flashback Technologies, Inc. was granted 510(k) clearance by the U.S. FDA for this, now dubbed the CipherOx® CRI M1 monitor. The M1 is Flashback’s first commercial CRI product to market and commercial sales of the M1 are expected to begin soon. Read the press release here.